Small Molecules, Big Impact
At LOXIM pharmaceuticals, we don’t just manufacture molecules—we build the foundation for tomorrow’s treatments. Backed by over four decades of chemical expertise, we are enabling a new generation of life-saving medicines through precision manufacturing, sustainability, and partnership-driven growth.

What We Do
From lab-scale development to full-scale production, LOXIM supports pharmaceutical innovators across the globe in accelerating drug development while meeting the highest benchmarks of quality, safety, and compliance.
Why LOXIM Pharmaceuticals?
Chemistry That Delivers
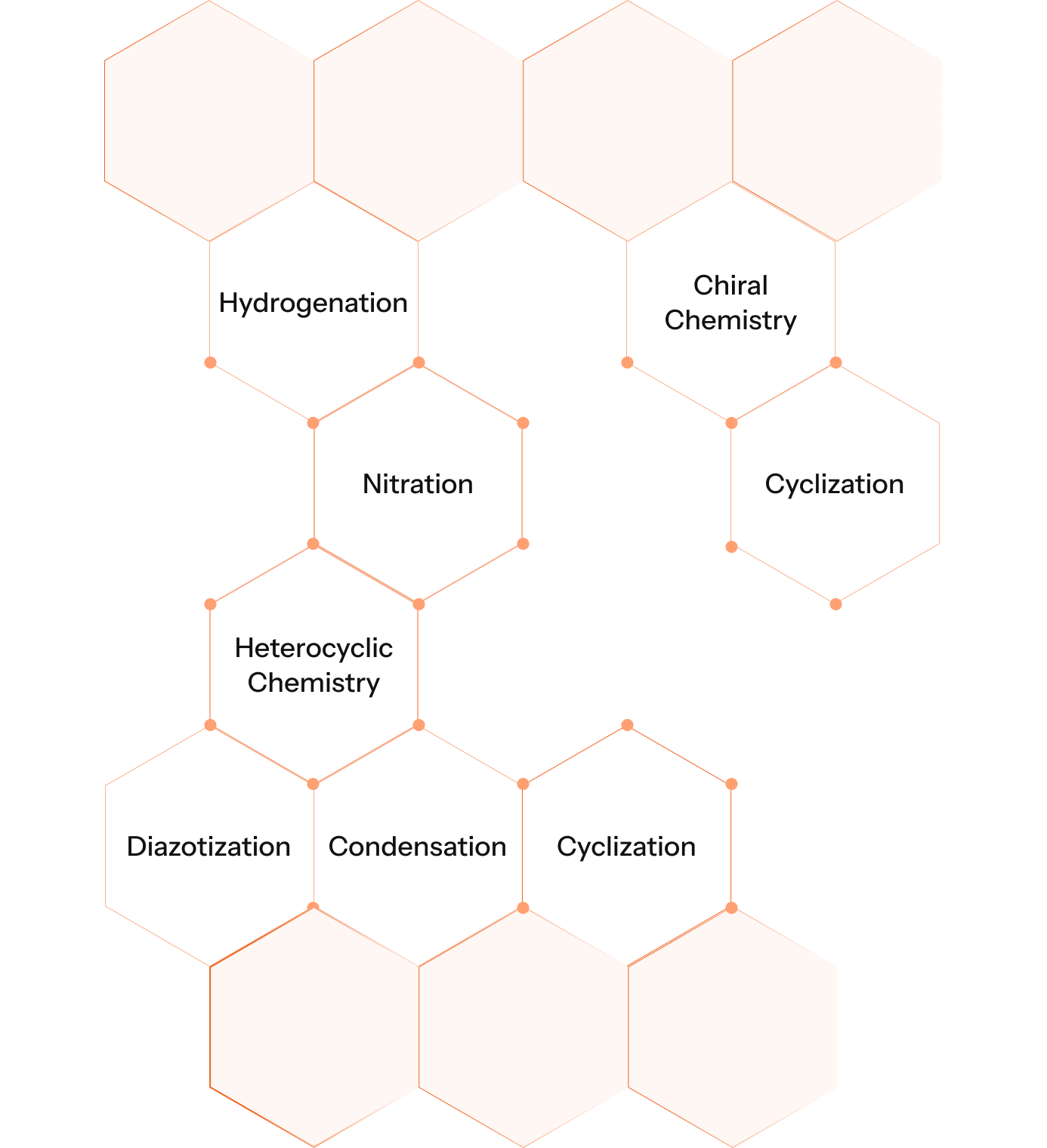
With a deep foundation in complex synthesis, we handle diverse chemical reactions including hydrogenation, oxidation, diazotization, condensation, chiral chemistry and high-pressure reactions with precision and safety.

cGMP-Compliant, Future-Ready Facilities
Our new 60,000 sq. m greenfield facility in Saykha, Gujarat is designed to global regulatory
standards, with a plant layout already approved by the local FDA, includes:
- Multi-purpose production blocks
- Kilo lab and pilot plant for seamless scale-up
- Hydrogenation and solvent recovery blocks
- Dedicated quality and regulatory units

Integrated R&D and Scale-Up
Our top-tier R&D infrastructure supports:
- Process development
- Analytical method development
- In-process control (IPC)
- Process optimization for yield and sustainability
- Seamless handover from kilo lab to commercial batches

Certifications & Regulatory Compliance
- ISO 9001:2015
- Local FDA-compliant layout
- Environmental clearance
- Upcoming certifications: WHO-GMP, ISO 14001, ISO 45001

Our R&D Capabilities
Innovation in Chemistry, Designed for Scalability and Compliance At LOXIM, research is more than just discovery — it’s the engine that drives our precision, safety, and regulatory compliance.

Advanced R&D Infrastructure
Our research centre features 9 fume hoods, a cleanroom, a kilo lab, and dedicated spaces for process and analytical development. The facility is equipped to handle diverse reaction types while maintaining strict standards of safety and quality.

Expertise in Complex Chemistry
Our team specializes in a wide spectrum of chemistries including heterocyclic synthesis, oxidations and reductions, asymmetric synthesis, and Grignard reactions. We also manage high-pressure reactions and those involving reagents like n-BuLi, LDA, DIBAL, and more — supporting the development of chiral molecules and IP-compliant synthesis routes.

Scalable & Sustainable Process Development
We develop cost-effective, non-infringing routes for APIs and critical intermediates that are environmentally safe and regulatory aligned. Our strong technology transfer team ensures smooth scale-up from lab to production, minimizing risk and ensuring robust processes ready for commercial rollout.

Analytical Development & Support
Our analytical lab is equipped with modern instrumentation including HPLC, GC, UV, IR, polarimeters, and auto-titrators. The lab supports method development, method validation, impurity profiling, stability studies (as per ICH guidelines), genotoxic impurity analysis, chiral method development, method transfer and regulatory documentation.

Quality and Regulatory Alignment
We follow current Good Laboratory Practices (cGLP) with rigorous documentation across all development phases. Our analytical and process teams work closely with regulatory and quality functions to support smooth filings and address customer and authority queries with precision.
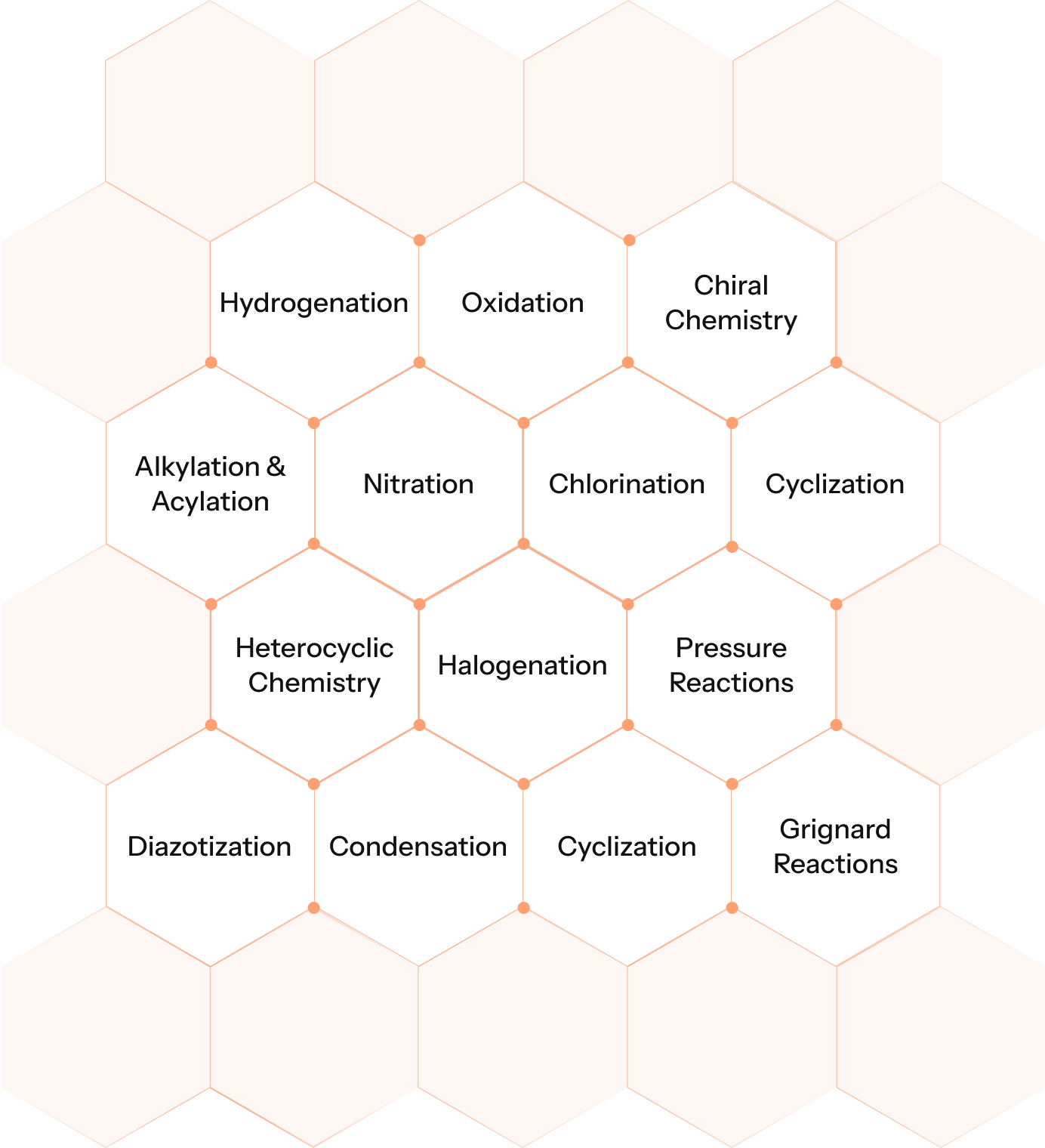
Our Key Reaction Capabilities
This broad toolkit enables us to deliver customised solutions for complex API, Intermediates, and KSMS.
Value We Offer
In-house Kilo Lab Capabilities
Robust Customer Support
Ease of Collaboration and Execution
Regulatory Readiness
FDA-Standard Manufacturing Readiness
Globally Aligned Quality Systems
Smooth R&D to Pilot Transfer
Sustainability by Design
At LOXIM, our values shape how we work—with care for people and respect for the environment at the core of everything we do.

Zero Liquid Discharge (ZLD) with ETP, NF-RO, and MVR

Natural Gas & Bio Briquettes replacing furnace oil

Optimized energy usage through efficient equipment

Water reuse & green spaces at all manufacturing units


Let’s Build
What Matters Together
Whether you're seeking a reliable intermediate and API supplier or a trusted partner for CMO & CDMO, LOXIM Pharmaceuticals is ready to collaborate with you.
Get in Touch